Introduction
Conservation of electric charge is one of the basic laws of nature. During the 18th century, one of the physicists named as Benjamin Franklin discovered the electric charge. He started the study of electricity in 1740 itself. For this discovery he went through some experiments and found the existence of the positive charge and negative charge. But this concept came into practice only when the batteries were introduced.
Definition of Electric Charge
Electric charge is one of the basic physical properties of matter, which is carried out by some elementary particles like electrons, protons, lepton, krypton and meson. Electric charge governs how these particles are getting affected by the electric or a magnetic field. Simply electric charge is defined as some amount of electricity that is held or carried out by some particles.
An electric charge will be created when electrons are removed or transferred into an object. Generally. electrons have negatively charged particles, when such particles are added to an object it becomes a negatively charged object. If we remove those electrons the object will become a positively charged object.
Explore our latest online courses and learn new skills at your own pace. Enroll and become a certified expert to boost your career.
Properties of Electric Charge
Additivity of Charges
Basically, electric charges are additive in nature. The total electric charge of a system should be equal to the algebraic sum of individual charges located in different places inside the system. This is also known as superimposition law for an electric charge.
Conservation of Electric Charge
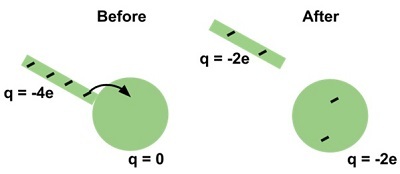
According to the principle of conservation of charges, we can neither create nor destroy electric charge. We are supposed to only transfer the charge from one body to another.
At the subatomic level, charges can be created. But always this creation will be done in pairs equally with positive and negative charges. Hence the total amount of the charges will remain as constant.
The formula for an electric charge is,
q=∫tftiIdtq=∫titfIdt
Where q = charge
ti = initial time
tf = final time
I = net outward current
dt = change in time.
Quantisation of Charge
Quantisation of electric charge means the charges will take up only the discrete values. Generally electric charges are quantised. In other words, the electric charge can only exist in nature in the form of the integral multiples of charge on one electron or proton. The magnitude of the charge is represented as e= 1.6 x 10-19 C. The S.I. unit of electric charge is Coulomb.
Invariant of Charge
The electric charge is always invariant, simply the charge does not change with respect to the change in velocity. According to the relativity theory, mass, time and the length will change with the change in velocity, but the charge doesn’t change.
Charges Exert Force
Like charges will repel each other, whereas unlike charges will attract each other. An electrically charged body will attract the lighter neutral body.
Types of Electric Charges
In general, there are three types of charges in the universe. They are referred to as positive charge, negative charge and the neutral charge. An atom consists of three major particles as proton, electron and the neutron.
Among these particles, an electron will carry the negatively charged particles, the proton will carry the positively charged particles whereas the neutrons will carry the neutral particles.
Hence, the electric charges are majorly referred to as having two kinds of particles as a positively charged particle and a negatively charged particle.
There are three methodologies used to charge or alter the charge of an object. They are friction, conduction and induction. With the help of these three methods one can able to charge the empty body with either positive charge or negative charge and also, we can alter the charge of an object.
How the Electric Charges are measured?
The S.I unit which is used to measure the electric charge is Coulomb. This was invented by the 18th century French Physicist Charles Augustin Coulomb. He developed the law, stating that the like charges will repel each other, while the unlike charges will attract each other, which was one of the major properties for the electric charges.
Coulomb did an experiment by bringing the similarly charged pith ball near any one of the needles. Then he determined the existence of the repulsive force between the balls by their separation function from this he came to conclude the law of attraction.
Generally, the quantity of electric charge can be measured in both direct and indirect methods. Directly it was measured using the electrometer, whereas indirectly electric charge is measured using the Ballistic Galvanometer.
The unit of the electric charge “Coulomb” is defined as an amount of charge transported by the current of one ampere in one second.
Coulomb’s Law
According to Coulomb’s law, the force of attraction and repulsion between the two charged bodies are directly proportional to the product of the charges and indirectly proportional to the square of a distance separated by the two charges.
The formula for the Coulomb’s law is
F=kq1q2r2F=kq1q2r2
Here, F = electric force
k = proportionality constant
q1 and q2 = point charges
r = distance between the two-point charges.
This law is basically used to calculate the electric force between the two charged particles. The electric force occurring between the two charged bodies at rest is called an electrostatic force or coulomb force.
Leave a Reply