Introduction
In accordance with a general conceptualisation, spectral series are referred to as a set of parallel lines that represent uniform speed and distance. In such cases, it has been observed that the wavelength of light can create a significant impact on the wavelength of these spectral lines. Based on this basic conviction, the present tutorial will define the spectral series of Hydrogen atoms. Moreover, the tutorial will include the formation of the spectral series along with the explanation of the Rydberg formula.
Spectral Series: Definition
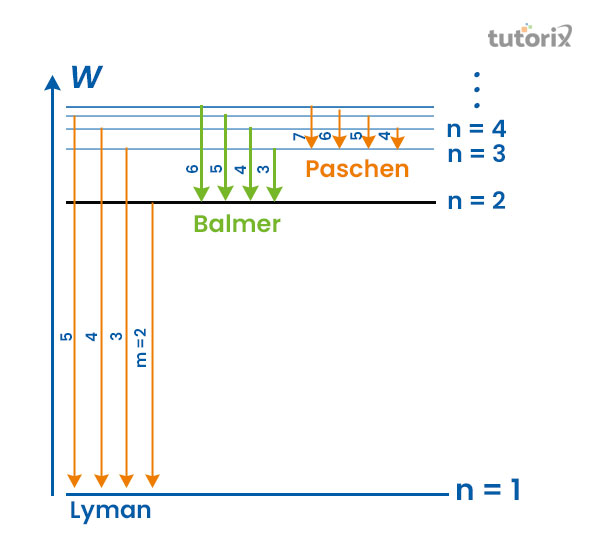
Figure 1: Spectral series
The easiest way of understanding the principle of the spectral series is considered the study of hydrogen atoms. The most basic atomic system found in nature is Hydrogen with one electron and one portion. An image forms whenever the light or radiation enters a device with a slit. This picture can be observed with the help of a spectroscope (Du et al. 2021). The spectral lines, formed by the wavelength of the light, are arranged in a parallel form next to each other with consistent spacing.
Explore our latest online courses and learn new skills at your own pace. Enroll and become a certified expert to boost your career.
Formation of Spectral Series
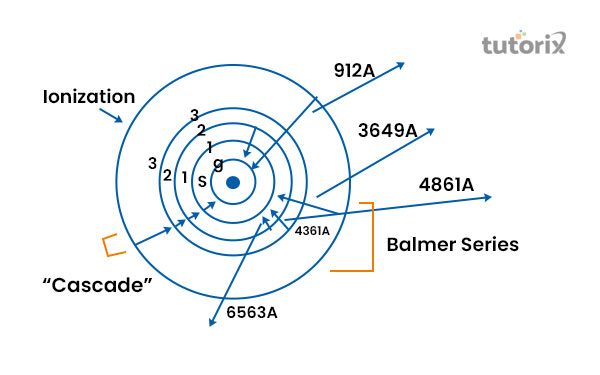
Figure 2: Formation of Spectral Series
In order to understand the formation of the spectral series, Bohr’s atomic model is generally used.
Being applied to the classical electromagnetic theory, this model is used in order to explain the set of energy levels that has been enclosed by each atom (McGuire et al. 2021).
Energy states are generally named on the basis of the quantum numbers. A release of a photon with energy nh – nl is observed in the time when electrons jump to a lower energy state (nl) from a higher energy one (nh).
The production of the same energy phonon is witnessed in time when a transition happens within two energy states that are similar to each other because the associated energy in every state is fixed which helps to divide the spectral series into an equivalent series.
Rydberg formula
Figure 3: Rydberg formula
The atomic Hydrogen is considered responsible for putting on the view of The emission spectrum. This specific spectrum is found to be made up of different numerical spectral series.
Once, the energy levels reach the excited state, a transition between energy levels is observed in the electrons of Gas. The wavelength of the spectral series can be calculated by the use of the Rydberg formula (En.universaldenker, 2022).
The difference between energy levels that are observed in Bohr’s model and the wavelengths of the spectral lines of the emitted photon is generally represented by the mathematical representation of Rydberg formula.
The mathematical representation of this formula is 1λ=RZ2(1n21−1n22)1λ=RZ2(1n12−1n22). In this formula, the letter R is considered Rydberg constant with the value of 1.09737107 m-1. λ represents wavelength and Z is the atomic number.
Types of Spectral Series
Lyman series
This particuler series is named after Theodore Lyman as he was the founder of that series. In accordance with Bohr’s model, when transitioning of electoral is observed to the level of nl = 1 from higher energy states, the appearance of the Lyman series can be seen (Quimby et al. 2018).
Balmer series
Johann Balmer generally founds this specific series in the year 1885. The occurrence of the Balmer series can be observed in the time of shifting electrons from the energy level that is higher to the lower energy state (nl = 2) that are visible in the spectrum of electron (400nm to 740nm).
Paschen series
The name of series is named after Friedrich Paschen who found this spectrum in the year 1908. At the time of migration of the electrons to the lower energy states (nl = 3) from higher energy levels are considered as Paschen series (Ucolick, 2022). The wavelength of this series is in the infrared range of the electromagnetic spectrum.
Brackett series
Friedrich Sumner Brackett founds this specific series in the year 1922 therefore, this series is named after him. At the time when the electrons are started shifting from a higher energy state towards a lower one, the Brackett series appears.
Pfund series
This series is named after August Harman Pfund and it occurs when the electrons migrate to a lower energy state (nl = 5). The wavelength of this series is in all infrared part of the spectrum that is considered as electromagnetic.
Humphrey’s series
This series is funded by Curtis J Humphreys which occurs when the electrons migrate from a higher energy state to a lower one (nl = 6).
Conclusion
The tutorial has shed light on the representation of the concept of the spectral series that can be observed from different perspectives at different stages of the atom spectrum of hydrogen. In the tutorial, it has been included that in order to calculate the emission of the spectrum, the formula that has the mathematical expression like 1λ=RZ2(1n21−1n22)1λ=RZ2(1n12−1n22) is used. In this formula, the value of R is considered 1.09737 ✕ 107 m⁻¹. Moreover, the tutorial has explained several different types of spectral series.Introduction
In accordance with a general conceptualisation, spectral series are referred to as a set of parallel lines that represent uniform speed and distance. In such cases, it has been observed that the wavelength of light can create a significant impact on the wavelength of these spectral lines. Based on this basic conviction, the present tutorial will define the spectral series of Hydrogen atoms. Moreover, the tutorial will include the formation of the spectral series along with the explanation of the Rydberg formula.
Spectral Series: Definition

Figure 1: Spectral series
The easiest way of understanding the principle of the spectral series is considered the study of hydrogen atoms. The most basic atomic system found in nature is Hydrogen with one electron and one portion. An image forms whenever the light or radiation enters a device with a slit. This picture can be observed with the help of a spectroscope (Du et al. 2021). The spectral lines, formed by the wavelength of the light, are arranged in a parallel form next to each other with consistent spacing.
Explore our latest online courses and learn new skills at your own pace. Enroll and become a certified expert to boost your career.
Formation of Spectral Series

Figure 2: Formation of Spectral Series
In order to understand the formation of the spectral series, Bohr’s atomic model is generally used.
Being applied to the classical electromagnetic theory, this model is used in order to explain the set of energy levels that has been enclosed by each atom (McGuire et al. 2021).
Energy states are generally named on the basis of the quantum numbers. A release of a photon with energy nh – nl is observed in the time when electrons jump to a lower energy state (nl) from a higher energy one (nh).
The production of the same energy phonon is witnessed in time when a transition happens within two energy states that are similar to each other because the associated energy in every state is fixed which helps to divide the spectral series into an equivalent series.
Rydberg formula

Figure 3: Rydberg formula
The atomic Hydrogen is considered responsible for putting on the view of The emission spectrum. This specific spectrum is found to be made up of different numerical spectral series.
Once, the energy levels reach the excited state, a transition between energy levels is observed in the electrons of Gas. The wavelength of the spectral series can be calculated by the use of the Rydberg formula (En.universaldenker, 2022).
The difference between energy levels that are observed in Bohr’s model and the wavelengths of the spectral lines of the emitted photon is generally represented by the mathematical representation of Rydberg formula.
The mathematical representation of this formula is 1λ=RZ2(1n21−1n22)1λ=RZ2(1n12−1n22). In this formula, the letter R is considered Rydberg constant with the value of 1.09737107 m-1. λ represents wavelength and Z is the atomic number.
Types of Spectral Series
Lyman series
This particuler series is named after Theodore Lyman as he was the founder of that series. In accordance with Bohr’s model, when transitioning of electoral is observed to the level of nl = 1 from higher energy states, the appearance of the Lyman series can be seen (Quimby et al. 2018).
Balmer series
Johann Balmer generally founds this specific series in the year 1885. The occurrence of the Balmer series can be observed in the time of shifting electrons from the energy level that is higher to the lower energy state (nl = 2) that are visible in the spectrum of electron (400nm to 740nm).
Paschen series
The name of series is named after Friedrich Paschen who found this spectrum in the year 1908. At the time of migration of the electrons to the lower energy states (nl = 3) from higher energy levels are considered as Paschen series (Ucolick, 2022). The wavelength of this series is in the infrared range of the electromagnetic spectrum.
Brackett series
Friedrich Sumner Brackett founds this specific series in the year 1922 therefore, this series is named after him. At the time when the electrons are started shifting from a higher energy state towards a lower one, the Brackett series appears.
Pfund series
This series is named after August Harman Pfund and it occurs when the electrons migrate to a lower energy state (nl = 5). The wavelength of this series is in all infrared part of the spectrum that is considered as electromagnetic.
Humphrey’s series
This series is funded by Curtis J Humphreys which occurs when the electrons migrate from a higher energy state to a lower one (nl = 6).
Conclusion
The tutorial has shed light on the representation of the concept of the spectral series that can be observed from different perspectives at different stages of the atom spectrum of hydrogen. In the tutorial, it has been included that in order to calculate the emission of the spectrum, the formula that has the mathematical expression like 1λ=RZ2(1n21−1n22)1λ=RZ2(1n12−1n22) is used. In this formula, the value of R is considered 1.09737 ✕ 107 m⁻¹. Moreover, the tutorial has explained several different types of spectral series.
Leave a Reply