Introduction
A body of matter which is confined in a particular space by the boundaries is called a thermodynamic system. They have definite permeability. According to their boundaries they may be classified as closed systems, open systems, and isolated systems. In an isolated system, there is no transformation of energy or matter across the boundaries of the system. There is no work done on the system.
In a closed system, there is a transformation of energy but the matter cannot be transferred across the boundaries. In an open system, there is a free exchange of energy and matter across boundaries. Thermodynamics comes under the branch of physics which discusses heat, work, and temperature and their relations with energy, entropy, and some other physical properties of matter.
What is Latent Heat?
When a physical state of the substance changes without changing its temperature by absorbing or releasing energy. This energy is called latent heat. The latent heat concept was first given by the British chemist Joseph Black. To record the latent heat value he first used calorimetry. It is the work or energy that is needed to overcome the force between the atoms or molecules in a material. Generally, molecular vibrations are greater in gas. If heat is given to water the vibration will increase. Thus the applied energy changes the mobility of the molecules. This is called latent heat.
Explore our latest online courses and learn new skills at your own pace. Enroll and become a certified expert to boost your career.
Types of Latent Heat
This heat is conveyed in a system in three ways. They are latent heat of vaporization, latent heat of sublimation, and latent heat of fusion.
Latent Heat of Fusion
The quantity of heat energy that is grasped or emitted by the matter for the transition of solid to liquid is known as latent heat of fusion. The energy applied at air pressure melts the matter from solid to liquid. The change in enthalpy of the material which dissolves is the latent heat of fusion. Example ice gets melted by absorbing the heat.
Latent Heat of Vaporization
The quantity of heat energy that is grasped or emitted by the matter to transfer the matter from liquid to gas is the latent heat of vaporization. The energy applied to the matter at constant temperature for the transformation of the state of matter is the latent heat of vaporization. Here the latent heat is accompanied by no change in temperature but only the change in condition.
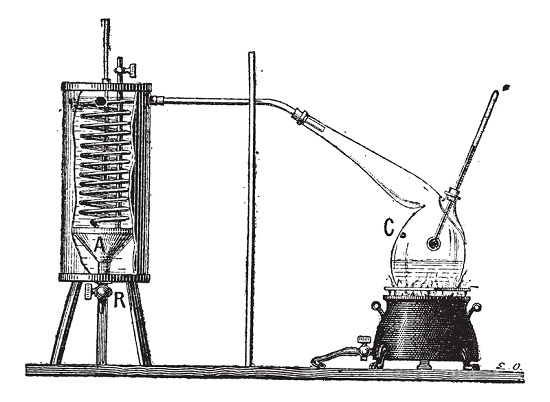
Fig:1 Apparatus for Measuring the Latent Heat of Vaporization of a Liquid, vintage engraving
Latent Heat of Sublimation
The quantity of energy that is grasped or released by the matter for the transition of solid directly to the gaseous state skipping the intermediate state. This energy is the latent heat of sublimation. The energy applied to the matter at a constant temperature for the transformation of solid into gas is called the latent heat of sublimation. For example, it is 2,838 kJ/kg for ice at 0°C.
Specific Latent Heat
The quantity of energy that is needed to change the state of one kilogram of material is the specific latent heat of the material.
The specific latent heat is mathematically expressed as,
L=QML=QM
Q denotes the energy absorbed or emitted by the material
M denotes the mass of the substance.
Specific Latent Heat of Water
When thermal energy is applied to the water then the temperature of water increases and it attains its boiling point of 100°C and stays constant. Then more energy from the source makes the water convert into steam.
TheenergydeliveredtothewaterQ=poweroftheenergysourcextimeTheenergydeliveredtothewaterQ=poweroftheenergysourcextime
Q=P×TQ=P×T
By knowing the power of the source the energy given to water is calculated.
Thespecificheatofwater=energyabsorbedMassofwaterThespecificheatofwater=energyabsorbedMassofwater
L=QML=QM
Latent Heat Capacity of Water
The material changes its state when it is heated by an external source. The temperature of the material remains constant until it changes its state. The transition of material from one state to another needs more energy.
When the water is heated, its temperature gradually increases up to 100°C. and remains constant until the whole water gets boiled. Similarly, when the water is cooled, it cools down up to 0°C and is constant until the whole water freezes. Not only for water all matter the temperature remains constant until the whole substance changes its state. because the energy must be used to change the state of the material from one form to another.
Leave a Reply