Introduction
Van der Waals Equation is commonly known as the Van der Waals Equation for real or a mole of gases that does not follow the law of ideal games. As per the law of ideal gases that are PV = nRT, where P refers to the external or internal pressure applied to the gases, V stands for the volume, and n denotes the number of the moles. In this formula, T denotes the temperature and R stands for the universal gas constant.
What is Van der Waals Equation?
Van der Waals Equation in terms of real gases denotes the states of the equation. In terms of real gases, the ideal gas behaviour does not follow the ideal gas equation in the context of pressure for the temperature. Derivation of the ideal gases in terms of applied pressure can be studied by plotting a graph that represents the opposition of pressure as well as volume curve in a constant temperature namely the law of Boyle.

Figure 1: Pair potential interaction of Van Der Waals Equation
The compressibility factor of the ideal gas behaviour is denoted by Z and can be present with the formula Z = PV/nRT. In terms of ideal gases, the compressibility factor is one but in the case of real gases, Z is not equivalent to one (Evarestov & Kuzmin, 2020). When the value of Z is more than one then the derivation of real gases is positive is Z>1, but when the value of Z is less than one then the derivation of real gases is negative which is Z
Explore our latest online courses and learn new skills at your own pace. Enroll and become a certified expert to boost your career.
Derivation of Van der Waals Equation for a mole of gas
According to the law of gases, the equation is PVm = RT but according to the Van der Waals Equation, it is C = Na/ Vm (Fedorov et al. 2018). in this formula, the net force that acts mainly on the molecule surface helps to pull out the container which is directly proportional to the density number.

Figure 2: Derivation of Van der Waals Equation for a mole of gas
Atm lit2 mol-2 and litre mol-¹ are the unit of Van der Waals Equation. At the low pressure, the compressibility factors that affect this equation is also presented as (P + a/V2) ( V – b) = RT but in most cases, b is neglected then the equation becomes (P + a/V2) ( V) = RT. in the case of high pressure, the compressibility factors present through the equation with (P + a/V2 ) ( V – b) = RT where a/V2 is generally neglected with the comparison of pressure, then the equation is (P) ( V – b) = RT
Derivation of Van der Waals Equation for real gases
In terms of real gases mostly the Van der Waals gas equation is applied. The real gas’s volume is mainly represented as (Vm – b), in which b is considered as the entire volume consumed by a unit of mole (Kontogeorgis, Privat & Jaubert, 2019). The Van der Waals gas equation for the real gases is P(Vm – b) = nRT in which V can be substituted by Vm, the volume of a molar of gas, r represents the universal gas constant and T denotes the temperature.
Van der Waals Equation application on compressible fluids
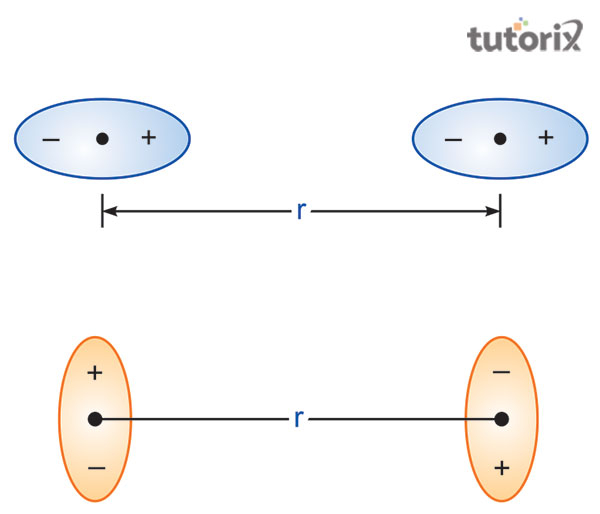
Figure 3: Interactions of Van der Waals gas equation
In the application of the Van der Waals gas equation on the compressible fluids the concept of the fluid composition should be clear. A polymer which is a compressible fluid has a particular volume as well as this volume is generally expressed with the formula (p + A)(V – B) = CT in which V refers to the particular volume, P stands for the pressure, T denotes the temperature and A, B, C are the parameters.
Advantages and disadvantages of Van der Waals Equation
Advantages
- It can foretell the actual behaviour of real gases better as well as accurate compared to the ideal gases.
- This equation is very much applicable for the fluid gas spite (Kontogeorgis, Privat & Jaubert, 2019).
- The cubic equation provides three different types of volume that are applicable for measuring the volume at as well as below the gas’s critical temperature.
Disadvantages
- The equation is just getting the authentic number of real gases that are above the critical temperature
- The critical temperature which gets in the below temperature is also accepted.
- In terms of the transition phase of different gases this equation failed.
Conclusion
This equation is mainly derived from the equation of ideal gases which states that there are a few point masses that are present in the gas molecule that undergo elastic collisions through the gases. Van der Waals Equation is used severally because the equation of gases is not able to explain the behaviour of the real gases properly and to derive the gas’s physical state this equation is used.
Leave a Reply