Introduction
In recent times, most electronic devices have a battery that helps transform the chemical energy into the electrical energy. Primarily the battery of an electric device can be classified into two differed types primary cell or battery and secondary cell or battery. A battery refers to a collection of a single or more than one cell that goes under the different chemical reactions for creating an electric flow to other devices of the machine. Recently, different types of technologies are used in making an electric battery like breakthrough technologies.
Battery and its applications
Battery refers to a small type of essential component of the electric device that guides to operate of the working process of the other machinery particles of a device. Some batteries can be rechargeable and they are used in different sectors housing complex, making of health instruments, the medical industry, logistics as well as construction sites. Besides this, in the field of firefighting, military and even emergency cases the application of batteries is commonly seen (Tamilselvi et al. 2021). For example, for housing purposes batteries are used in a torch, inverters and most electronic devices. In the medical sector, batteries run the different pathological devices. In the military field, the battery is very useful for communicating with radio technology.
Explore our latest online courses and learn new skills at your own pace. Enroll and become a certified expert to boost your career.
The components of a battery
There are different types of components present in a battery such as an anode, cathode as well as electrolytes. The anode of the battery denotes the negative electrode while the cathode stands for the positive electrode. Anode produced electrons to an electronic circuit in which a battery is connected. After creating a connection an electron creation is then initiated that causes a difference between the electrodes. In different types of batteries, the arrangements of these three components of the batteries are different (Löbberding et al. 2020). Other components of the batteries are the power capacity, power capability, voltage capacity, power density, and shelf life.
Different types of batteries with their applications
Batteries are mainly classified into two different categories like Primary and secondary cells.
Primary cells/battery
Primary cells have a higher density with slower dischargeable. It has also higher internal resistance with an irreversible chemical reaction.
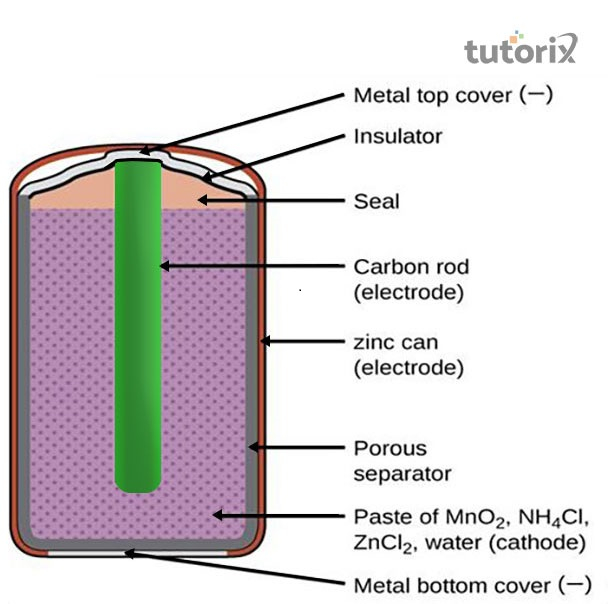
Figure 1: Construction of the primary battery
The design of this type of battery is smaller, lighter as well as thinner that is easily portable. The cost of this cell is also lower than the secondary cells (Halimah, Rahardian & Budiman, 2019). The redox reaction of this cell at the anode is Zn(s)→Zn2+(aq)+2e–Zn(s)→Zn2+(aq)+2e– while at cathode the equation changed to Zn(s)+2NH+4(aq)+2MnO2(S)→[Zn(NH3)2]2+(aq)+Mn2O3(S)+H2O(l)Zn(s)+2NH4+(aq)+2MnO2(S)→[Zn(NH3)2]2+(aq)+Mn2O3(S)+H2O(l)
Secondary cells/battery
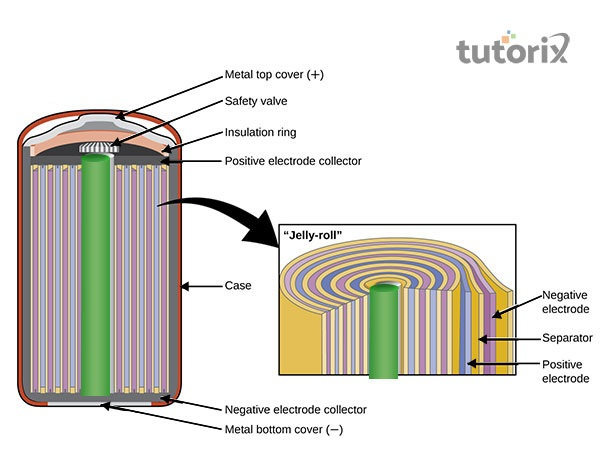
Figure 2: Construction of the secondary battery
The secondary battery consists of a lower energy density that is mainly made with wet cells as well as molten salt. It also comprises lower internal resistance, a reversible chemical reaction and the design of this type of battery is complex as well as heavier than primary cells (Thirugnanam et al. 2018). The redox reaction of this cell at the anode is Pb→Pb2++2e–Pb→Pb2++2e– and Pb+SO2–4→PbSO4(electrode)+2e–Pb+SO42–→PbSO4(electrode)+2e– while at the cathode the equation changed to 2e–+PbO2+4H+→Pb2++2H2O2e–+PbO2+4H+→Pb2++2H2O and 2e–+PbO2+4H++SO2−4→PbSO4(electrode)+2H2O2e–+PbO2+4H++SO42−→PbSO4(electrode)+2H2O. The secondary batteries are classified into two different categories rechargeable and non-rechargeable batteries.
Classification of secondary batteries
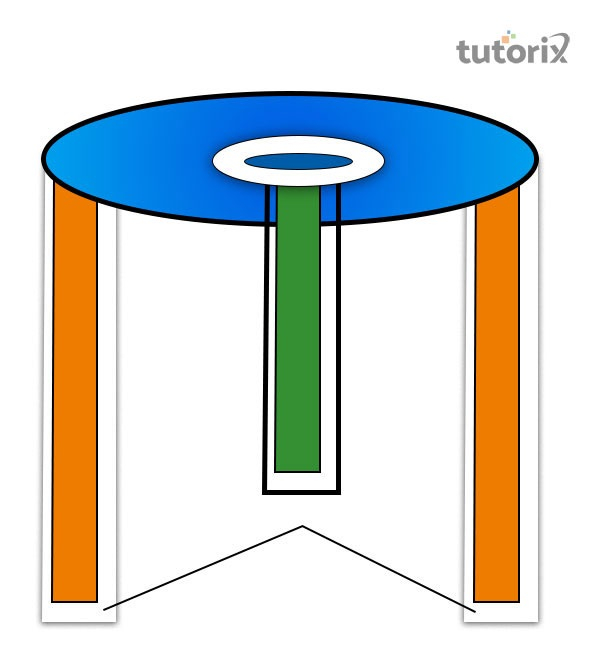
Figure 3: Construction of a dry cells
Rechargeable batteries have also different types like Lead-acid batteries, is a cheap battery mostly seen in cars, heavy machinery, UPS, and vehicles. The nominal voltage of these types of batteries starts from 2V to 24V and the power density is 7 Wh/Kg.
Secondary cells/battery
Lead-acid batteries
Advantages
- Cheaper in cost
- It is easily rechargeable
- Consists higher power capacity
Disadvantages
- Heavier in weight
- Consumed more space
- Lower power density
Li-ion batteries are made of Lithium and include advanced technology. The nominal voltage of these types of batteries starts from 3.7V and the power density is 126 Wh/Kg (Helbig et al. 2018).
Advantages
- It is lighter in weight
- Higher C-rating facility
- Higher cell voltage
- Consists higher power capacity
Disadvantages
- Unavailability of battery protection circuit
- Expensive than other types of batteries
- Great chance for short circuit
Non-rechargeable batteries are also classified into different categories such as Alkaline batteries and Coin cell batteries. Alkaline batteries consists of the chemical construction of Zinc and Manganese dioxide. The power density of this type of battery is 100 Wh/Kg. The application of this type of battery can be seen in electric torches, different types of remotes, wall clocks and many other daily life products.
Advantages
- It has a lighter cycle life
- Lower leakage chance
- Smaller in size and higher efficiency
- Lower internal resistance power and easy to port
Disadvantages
- Expensive than other types of batteries and is mostly unavailable in the regional market.
Leave a Reply