Introduction
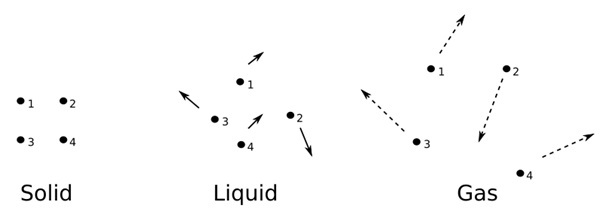
- Solids are densely packed formations that cannot be easily influenced by external factors.
- They have a set volume, mass and form as well. However, solids never flow. The chemical and physical properties of solids are studied in solid-state chemistry & solid-state physics.
- Nature has four different states of matter. Based on their intermolecular interaction as well as the nature of the component particles, they are classed as solids, gases, liquids & plasma.
Solid-liquid-gas.jpg: Sadi Carnot derivative work: Dave.Dunford (talk) 13:43, 15 December 2010 (UTC), Solid-liquid-gas, marked as public domain, more details on Wikimedia Commons
What is a Solid State?
- Solids are one of the 3 forms of matter that have a firmly packed structure and a set volume and structure.
- Chemical bonding, which keeps the molecules together, is responsible for the hard structure because the molecules are firmly bonded, their kinetic energy is smaller than those of gases and liquids.
- This property allows them to resist the activities of external factors and therefore maintain a constant volume and form, as opposed to gases and liquids which are very volatile.
Explore our latest online courses and learn new skills at your own pace. Enroll and become a certified expert to boost your career.
Characteristics Of Solids
- Because significant intermolecular interactions keep the component particles of matter together, solids have a particular weight, volume, and form. At low temperatures, the intermolecular force tends to control heat energy, and solids remain in a stable state. The volume and mass of a liquid and a solid state are the same for a given substance.
- Conductivity, density, and optical transmission are just a few of the numerous characteristics of solids.
- Common table salt, water ice, dry ice, rock, most metals, & wood are instances of solids. The atoms or molecules in a solid gain kinetic energy when warmed.
- There are 2 types of solids: crystalline & amorphous. The most frequent form of solid is crystalline solid. They are distinguished by a consistent crystalline arrangement of atoms that confers long-range order. This long-range organization is absent in non-crystalline or amorphous substances. Crystalline solids are those that have their atoms, ions, or molecules arranged in a regular, well-defined pattern.
- Molecular, Ionic, covalent, and metallic solids are the four primary kinds of solids. Ionic solids are made up of ions with positive and negative charges that are bound together by electrostatic interaction; the bonding strength is represented in the lattice energy. Ionic solids have a high melting point and are quite hard.
- Some chemicals create crystalline solids, which are composed of particles in a highly structured manner; others form amorphous solids, which have an unorganized inner structure.
- Mechanical properties of solids explain properties such as deformation and strength resistance. Plasticity, abrasion, ductility, malleability, and toughness are examples of mechanical qualities.
- A solid is distinguished by its structural stiffness & resistance to force applied to its surface. A solid substance, unlike a liquid, does not move to take on the shape of its vessel, nor does it swell to fill the full available capacity as gas does.
- Solids have distinct forms & dimensions and are not compressible in any way.
- The ions, atoms, or molecules in crystalline materials are organized in an organized & symmetrical arrangement that is repeated throughout the crystal. A unit cell represents the smallest recurring structure of a solid, similar to a brick in a wall. A crystal lattice is a network formed by unit cells.
Uses
Solid-state materials are widely used everywhere around us. Among the most important are −
- Mobile phones and personal computers are examples of electronic devices.
- Lasers and fibre optics are examples of optical devices.
As a result, the entire concept of modern invention is based on solid-state physics concepts.
Applications
- Commonplace items like wiring in a building and window panes, as well as the magnet in a fridge, are heavily reliant on solid-state.
- Logic and memory bits are made of silicon, which is solid.
Leave a Reply