Introduction
Before getting an idea of the relationship that exhibits between the electron volt and joule, we first need to define what is defined as units. In Physics, the term unit is used as a standard of measurement for several physical quantities. Units are used in building up a relationship among the different measures of equal quantities. In any scientific method, Joule states the SI” or Standard International unit for providing a standard measurement of amounts of energy, whereas, electron volt states to provide the value of energy gained.
Definition of Electron Volt
Electron volt, can be defined as the unit that is required to provide a description of the amount of net energy that is gained by an electron. This unit is only used when the electron is made to accelerate under certain special condition that includes a potential difference of measurement of 1 volt (Rapidtables.com, 2022).
However, volt (V) or (E) is the SI unit that is used to define and measure the differences between electric potential or EMF or electromotive force between two points. The formula for volt is defined as the multiplication of current to the resistance.
Therefore, electron volt in simpler words can be stated as the equal amount of energy received by an electron that is carrying one unit of electron charge, while electrical potential within an electron increases by 1 volt (Science.nasa.gov, 2022). Thus, the energy received is said to be 1 eV. Although the symbol used for electron volt is said to have been applied in higher physics. Several forms of electron volt are noticed, including, KeV, TeV, GeV, and MeV.
Explore our latest online courses and learn new skills at your own pace. Enroll and become a certified expert to boost your career.
The formula of an electron volt
The formula that established a relationship between electron volt to that of volt can be written as one volt is equal to 1.0E – 18EV ”. This is because ten to the power eighteen volts is defined as 1 exa volt, that means, I exavolt = 1018 Volts.
First chemical battery
The invention of the first chemical battery provided the concept of voltage which was by the Lombard physicist Alessandro Volta. More specifically he invented the voltaic pile. The diagram below represents the experimentation.
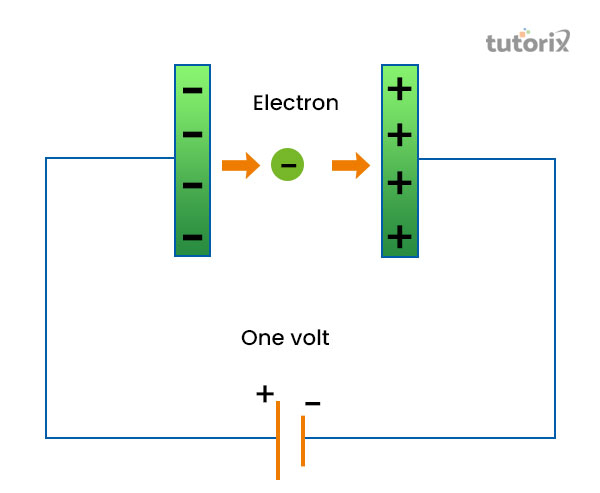
Figure 1: Electron Volt definition
In this experiment, a parallel plate capacitor is being used and as the process starts the electron becomes active in the negative plate and accelerates towards the positive plate that is from the presence of the parallel plate for capacitors. This is because the positive plate is at a higher potential of one volt (Lu et al. 2022). Therefore, the total work done by a free electron from one point to the other point tends to have a potential difference of 1 volt, and is defined as 1 electron volt. Thus, 1eV = 1 volt multiplied by charge acquired by an electron, implies, 1.6 * 10-19 [1V = (1J/1C)].
Definition of Joule
Joule is defined as the SI unit for measuring the amount of work done or energy. However, one joule can be stated as the net amount of energy that is exerted at the time of application of one-Newton force over a displacement that occurs through 1 meter (Electronics-tutorials.ws, 2022). It is also stated that one joule is equal to radiation or dissipation of 1 watt of power for about a second.

Figure 2: Joule definition
In the above image, the SI unit of energy is shown. In the above diagram, the transference of energy is occurring by the force of one Newton that is acted upon the object, thereby displacing it in the same identical direction along with a distance of 1 meter (Techtarget.com, 2022). Joule is not only defined as transfers of energy but also the work done by the object.
Explaining the formula of 1 unit of Joule
The formula for explaining 1 unit of joule is stated as, 1J = 6.2415 * 1018 eV (Mrsolar.com, 2022). This further defines the energy or total work done in displacing the object along with a distance of 1 meter in the identical direction.
Conversion
Conversion of Electron Volt to Joule
The conversion of electron volt is stated below.
Therefore,
- 1 eV equals to 1.60218 * 10-19 J,
- 2 eV equals to 3.2044 * 10-19 J,
- 3 eV equals to 4.8065 * 10-19 J and so on.
Conversion of Joule to Electron Volt
The conversion of joule to electron volt is shown below.
Therefore,
- 1 J equals to 6.242 * 1018 eV,
- 2 J equals to 1.248 * 1018 eV,
- 3 J equals to 1.872 * 1018 eV and so on.
Application of the relationship of Joule and Electron volt
It needs to be noted that the conversion of joule to electron volt and vice versa is quite necessary for solving any kinds of problems associated with the fields of nuclear physics and atomic energy plants. Moreover, the conversation table provides easy access to solving the problems associated with work done and the transference of energy (Science.nasa.gov, 2022). The relationship is quite essential as it provides easy convenience to understand the concept associated with the units of joule and electron volts.
Leave a Reply