Introduction
Thermodynamics is a study that establishes a relationship between heat and work. To understand it well, the concept of two terms- entropy and enthalpy has to be deeply dug in. In this article, the basic definitions of entropy and enthalpy, as well as the differences will be discussed in an ordered way. In very simple words, the entropy is the measure of randomness; on the other hand, the enthalpy represents the total heat of the system.
What is Entropy?
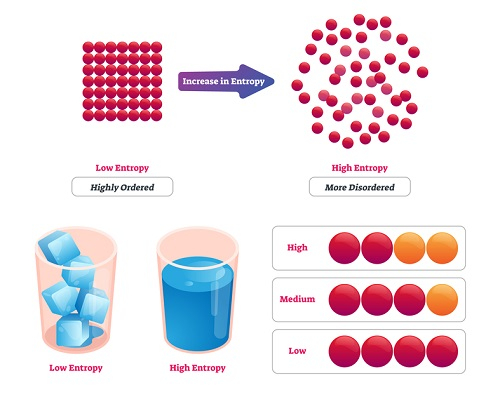
Entropy is a measurable physical property that represents the degree of disorder of a system. Any kind of matter or medium- fluid or solid, is made up of molecules. The more disordered and random the molecules are, the more will be the entropy. And hence, the part of heat which may be converted into work will not be much. In this way, we may say that entropy is a function of the heat quantity. Initially, the concept was coined with the name ‘heat potential. Later, Clausius, in his statement of the Second Law of Thermodynamics, it was defined it as the ratio of the very small change in the heat to the instantaneous temperature for a reversible process.
ΔS=ΔQTΔS=ΔQT
If the randomness of the molecules is less, the change in entropy will also be less. Hence the solids usually have less entropy than fluids. The entropy of the system, plus the surrounding, always increases. This implies that the entropy of the universe is always increasing.
Examples
Various instances of entropy changing can be seen around us. In daily life, we see evidence that the universe always tends toward the way where there is an increase in the entropy, i.e. the randomness. A few examples from day-to-day life can be listed as follows −
- Osmosis − When we light an incense stick in a closed calm room, the smoke produced always tends to spread out. It expands on its own as the molecules gain randomness and disorders. This is an example of the entropy increasing. We shall never see the smoke being settled and concentrated at a place on its own.
- Dissolving also leads to increased entropy. A solid is actually in a much-ordered state, on dissolving, goes into a more disordered one. Dissolving sugar in water increases the energy of the system as the randomness of the system increases and hence the entropy also increases.
- A campfire is also an example of entropy. The fuel-which is usually the solid wood, paper, or straw, burns and turns into ash which is much disordered. In addition, smoke and various gases like carbon dioxide are released. The atoms spread out in an expanding form, with increasing disorder, and hence the entropy is said to have increased.
- The phase change processes from one state to the other also bring the change in the entropy. The ice cube, in solid-state, has more orderness and hence more entropy than the water after the process of melting. The universe on its own will never push the process of freezing i.e the decrease in entropy.
Explore our latest online courses and learn new skills at your own pace. Enroll and become a certified expert to boost your career.
What is Enthalpy?
Enthalpy is the property of a thermodynamic system, which represents the total heat change in the system. To the First Law of Thermodynamics, it’s the sum of the internal energy and the product of pressure and volume. It is very important to know how much enthalpy has changed in a chemical reaction. We cannot measure the total enthalpy of the system directly because there are some unknown parameters in internal energy. Instead, we measure the change in the enthalpy for our simple to understand the process well. Mathematically, it can be represented as
H=U+PVH=U+PV
Here, U is the internal energy, P is pressure, and V is the volume of the system. Further, measuring the change in enthalpy also helps us to figure out if the reaction was endothermic (heat absorbed) or exothermic (heat released). Another important thing to note is that the order of the steps of a reaction, or the number of steps of a reaction, doesn’t affect the value of the enthalpy change of the reaction.
Examples
Enthalpy has many real-life applications and examples. A few of them can be listed as follows −
- Food brands and industries calculate how much energy the food is releasing by breaking bonds of glucose inside the body and hence keep a check on the number of calories in the food being sold.
- The automobile industries also check how much energy the engines are using for using up a certain amount of fuel. In simpler words, they use enthalpy and energy change to make efficient energy choices for the automobile and save money.
- Refrigerator compressors also the application of enthalpy. The refrigerant chemicals in the compressor get vaporized, and as a result, the heat is absorbed in an endothermic reaction.
Relation between Entropy and Enthalpy
With the help of the definitions discussed above, the terms entropy and enthalpy changes can be related as follows
When the change in enthalpy is negative: ΔΔH = -ve, we say that the heat is given to the surroundings, i.e. the exothermic reaction. It is a stable system and hence spontaneous. This means that the entropy of the surroundings also increases.
Whereas, when the change in enthalpy is positive: ΔΔH = +ve we say that the heat is added from the surrounding to the system, i.e. endothermic reaction. This means that the entropy of the surroundings decreases.
To perfectly relate the spontaneity of the reaction with the H and S, the following relation is used
ΔG=ΔH−TΔSΔG=ΔH−TΔS
This equation is known as the Gibbs Helmholtz equation. In this equation, ΔΔG is the change in free energy. For any spontaneous reaction to happen, ΔΔG is always negative.
Difference between Entropy and Enthalpy
| Sr. No. | Entropy | Enthalpy |
|---|---|---|
| 1 | It is a thermodynamic measurable property | It is a kind of energy |
| 2 | It is the measure of randomness | It is the measure of the total heat content of the system |
| 3 | A system always favours the maximum value of entropy | A system always favors the minimum value of enthalpy |
| 4 | Its unit is JK1JK1 | Its unit is Jmol1mol1 |
| 5 | It is denoted by symbol S | It is denoted by symbol H |
Leave a Reply